New method for cost effective classification of ultrafine 316L stainless steel powders
April 11, 2013
Stainless steel powders make up more than half of all the various alloy powders used in the global Powder Injection Moulding (PIM) industry, and there is increasing pressure on powder producers to supply the finest size fractions at competitive prices for the production of complex PIM components. Stainless steel powder for PIM is generally produced by gas atomisation using argon or nitrogen, or high pressure water atomisation, in order to achieve the desired spherical particle shape needed for PIM processing. However, both atomisation routes produce a relatively coarse powder from which the desired very fine powder needed by PIM producers (<40 µm and finer) have to be sieved out using air separation methods. This can make the required ultrafine stainless steel powders very expensive.
Now an interdisciplinary research project at the Division of Surface and Corrosion Science at the Royal Institute of Technology, Stockholm, Sweden, has developed technology whereby ultrafine 316L stainless steel powder particles lower than 20 µm can be economically separated from the bulk of the atomised stainless powder using magnetic separation. Dr Yolanda Hedberg and her colleagues in the paper “Ultrafine 316 L stainless steel particles with frozen-in magnetic structures characterised by means of electron backscattered diffraction” (Materials Letters 2011;65(14):2089-2092) found that using electron backscatter diffraction (EBSD), a technique traditionally used to assess structural information for massive materials, the smallest particle size fraction of gas atomised AISI 316L stainless steel powder (<4 µm) had a different crystallographic structure compared with larger size fractions of the same powder (<45 µm).
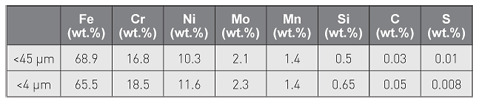
Despite similar chemical compositions, as shown in Table 1, the researchers found significant differences in crystallographic structure between the two particle size fractions of gas atomised 316L stainless steel powders (Fig. 1). Most of the ultrafine particles revealed a predominantly ferritic bcc structure (red) which existed as single crystals, whereas the larger size fractions generally showed particles having slightly smaller grain sizes compared with massive 316 L, but with the same austenitic crystallographic fcc structure (green) as in massive 316 L. As expected, ferrite was present to a very low extent (less than 10%) in the larger sized particle fraction and in massive sheet.
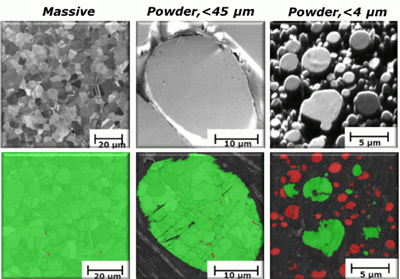
Fig. 1 Differences in microstructure (grain sizes) and crystallographic structure of 316 L as massive sheet (left), gas-atomised particles <45 µm (middle) and particles <4 µm (right), by means of SEM and EBSD showing the orientation of the different grains and different phases (green: austenitic and red: ferritic)
To explain why the thermo-dynamically unstable ferritic phase could be formed and predominate in most of the finest sized particles of the ultrafine 316L powder, particles of both size fractions were embedded, polished and etched to obtain further microstructural information via SEM imaging. The larger sized particles (<45 µm) revealed a dendritic microstructure, whereas the more rapidly cooled ultrafine sized particles (<4 µm) showed a cellular microstructure. The theoretical cooling rate of the larger sized particles was calculated based on the secondary dendrite arm spacing (DAS) value, which was estimated to be approximately 1 µm, which according to these calculations would correspond to a cooling rate in the order of 10,000 K s−1, or even some orders of magnitude higher than previously suggested in the literature. The cellular microstructure of the ultrafine sized powder suggests an even faster cooling rate, also indicated by the fact that most individual particles in fact existed as single crystals. Thermodynamic considerations suggest that the melt must be undercooled by at least 900 K to form ferrite in preference to austenite.
The researchers concluded that the differences in crystallographic structure and hence magnetic properties of the ultrafine metastable ferrite 316L stainless steel powder opens up the possibility for magnetic separation, without any expensive sieving or air classification steps, of the smallest particle size fraction obtained during gas atomisation of austenitic 316L stainless steel particles. They believe that the technology will help to lower the cost of producing PIM grade 316L powders, and potentially other ultrafine high-alloyed gas atomised powders, which in turn will help manufacturers achieve cost effective production of complex PIM components.
This research work has led to an application for a World Patent (WO 2012/125113) and the research team is keen to further exploit the invention with any interested parties.
Further information is available from Dr Yolanda Hedberg, email: [email protected]
News | PDF Store | Magazine Subscriptions | What is PIM? | e-newsletter