MIM parts help alleviate sleep related breathing disorders
January 5, 2016
The ability of CMG Technologies’ UK-based Metal Injection Moulding operation to produce high volumes of extremely small, complex parts has resulted in a contract with leading mandibular advancement manufacturer Somnowell Ltd.
According to UK health data, as many as one in four people in England snore regularly and one in ten of these have sleep apnoea, a potentially life threatening breathing disorder, meaning the market for oral devices that combat snoring and other sleep related breathing problems worldwide is potentially vast.
Established in 2010, Somnowell produces a range of oral appliances, also known as mandibular advancement devices, which are designed to alleviate snoring, sleep apnoea and teeth grinding (bruxism). The devices fit into the mouth and are worn whilst the user is asleep. They hold the jaw forward in the “recovery position” keeping the airway open.
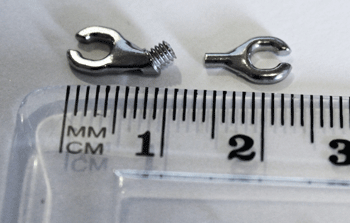
CMG Technologies has been commissioned to produce the end attachment claws of the arms that hold the top and bottom plates together whilst protruding the lower jaw relative to the upper. This results in the ultra slimline Somnowell Chrome, a tailor made device billed as ‘a lifetime solution and the most slim-line, comfortable and hygienic device available.’ Rachel Garrett, CMG’s Technical Sales and Marketing Director, stated, “The part was previously made from eroded wire using EDM (Electrical Discharge Machining). However because of the volumes needed and the complexity of the part, Somnowell Ltd wanted to find a more reliable, cost effective manufacturing method once the device had been proven.” Measuring just 10 mm in total and featuring an external thread and an internal access tube, the parts are now made from 316L stainless steel by CMG.
“One of our business associates recommended CMG to Somnowell Ltd and, following the production of successful prototypes, the first set of 500 parts was completed in time for the World Orthodontic Congress which took place in London at the end of September,” stated Garrett.
Using MIM to produce the parts, along with the other associated components, will reduce costs by approximately 40% when compared with typical EDM costs, with no compromise on quality.
Somnowell’s inventor, Visiting Professor Simon Ash, stated, “We launched the first Somnowell device back in 2005 and have since experienced a phenomenal take-up of our products with quite remarkable life changing clinical outcomes for patients from all over the world. The current Somnowell Chrome is the 4th generation device and is made from cast chrome cobalt alloy which is as hygienic as gold and far more comfortable and durable than plastic.”
“The volume of product necessitated the need for us to find an alternative way of manufacturing the part – not only did it need to be cost effective, it also had to be suitable for use in the mouth and capable of being replicated in volume production runs. We are anticipating sales in excess of 10,000 units in the UK alone, so finding the right supplier from the outset was vital.” Somnowell has recently achieved FDA approval in the USA for their product.