Malaysian team fabricates endoprosthetic hip stem using Metal Injection Moulding
February 23, 2018
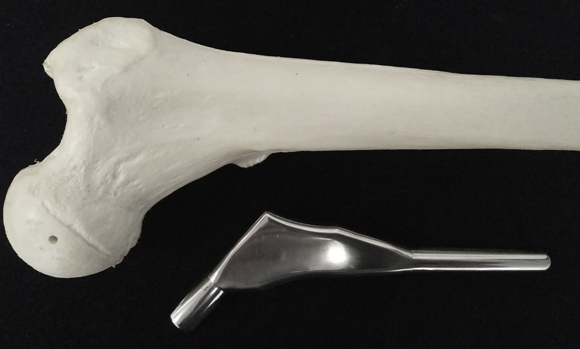
SIRIM Berhad’s MIM prosthetic hip stem
A recently completed research project undertaken by SIRIM Berhad (formerly the Standard and Industrial Research Institute of Malaysia) and the International Islamic University of Malaysia (IIUM) has successfully produced a prosthetic hip stem using Metal Injection Moulding.
The use of Co-based alloys for surgical applications is mainly related to orthopaedic prostheses for the knee, shoulder and hip as well as to fracture fixation devices. Joint endoprostheses (prosthetics which are implanted in the body) must meet extremely high requirements with regards to biocompatibility and corrosion resistance. CoCrMo has been widely used in endoprosthetics due to its mechanical properties, good wear and corrosion resistance and biocompatibility.
Dr Mohd Afian Omar, Principal Researcher, SIRIM Bhd, and colleagues are investigating the use of Metal Injection Moulding for the production of femoral hip stem implants with high surface quality. To manufacture the new implants, the team used CoCrMo alloy metal powder with a median particle size of 15 µm and a binder consisting of palm stearin and poly ethylene, with a powder loading of 65 vol.%.
According to the team, the hip stems were injection moulded using a vertical injection moulding machine with the nozzle temperature of 200°C. The hip stems were then debound using a combination of solvent extraction and thermal pyrolysis method, before sintering under vacuum at 1390°C. The results showed that the highest final sintered densities were about 98% of theoretical maximum value with good mechanical properties, including hardness of 42 HRC, elongation of 10% and UTS of 822 MPa, in line with standard ASTM F75.
To determine the potential toxicity and biocompatibility of the prosthetic, a cytotoxicity test of the hip stem was conducted, in which it was extracted for twenty-four hours in Minimum Essential Medium (MEM), and an extract prepared from the test material then placed on cell monolayers. The cells were examined for morphologic changes, malformation, degeneration and cytolysis to determine a toxicity score.
The cell morphology showed very little change from that of the control specimen, indicating that no morphological abnormalities took place. Cell growth towards the material demonstrated good biocompatibility, particularly on longer incubation times, which also indicated good material-tissue integration. A biomechanical study and preclinical test are expected to be conducted soon.
Email: [email protected]
www.sirim.my

















