Biocompatibility of 316L stainless steel vascular stents made by Metal Injection Moulding
October 7, 2022
Vascular stents are specially designed hollow metal mesh tubes that are inserted into a collapsed blood vessel via a catheter and permanently support the vessel’s walls to prevent another closure. They are reported to have excellent therapeutic effect and have a high rate of success in treating coronary and peripheral artery disease.
Currently, stents made by braiding or laser cutting techniques occupy most of the market share, but these techniques have certain disadvantages such as the high cost of equipment, high production costs, and poor geometric properties such as more nodes on the surface and a greater metal surface area, which can significantly increase the risk of thrombosis and restenosis rate after stent implantation.
Lower cost near net-shape processes are being explored for biodegradable stent materials but to date there have been very few reports on production of stents using Metal Injection Moulding (MIM) (see PIM International Vol. 6, No. 4, December 2012).
More recently, researchers at the Departments of Vascular Surgery, Second Xiangya Hospital, Central South University (Changsha), and the Department of Vascular Surgery, Fuwai Hospital (Beijing), in conjunction with the State Key Laboratory of Powder Metallurgy, and the School of Microelectronic and Materials Engineering, Guangxi University of Science and Technology (Liuzhou), all based in China, have been studying the use of MIM to produce stent scaffolds using 316L stainless steel powder.
The MIM 316L stents were implanted into the iliac arteries of adult dogs using a standard angioplasty technique. The MIM processing parameters, stent properties, status of experimental animals, and endothelialisation of implanted stents were investigated, and a molecular biology evaluation was carried out. The results of their investigation were published in the paper: ‘Biocompatibility of vascular stents manufactured using Metal Injection Moulding in animal experiments’ by Chang Shu, et al. in the Transactions of Nonferrous Metals Society of China Vol. 32, 2022, 569-580.
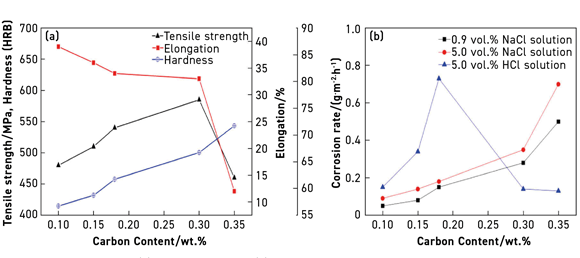
The authors reported that the gas atomised 316L stainless steel powder used in this study had a D50 = 12.5 µm particle size, and that this was mixed with a multicomponent binder comprising paraffin wax and low-density polyethylene (LDPE). In order to study the effect of carbon content fluctuations on the mechanical and corrosion properties of the sintered MIM 316L stainless steel, the authors added 0-0.4 wt.% graphite. Injection moulded tensile test specimens were subject to solvent debinding and thermal debinding followed by sintering at 1340°C and 0.1 Pa.
Results of the testing for tensile strength, elongation and hardness of the sintered 316L MIM samples with different carbon contents are shown in Fig. 1(a). It was found that increasing the carbon content from 0.1 wt.% to 0.30 wt.% saw an increase in the tensile strength from 480 to 585 MPa, which then decreased to 460 MPa at a carbon content of 0.35 wt.%. The hardness of the sintered sample increased with increasing carbon content, while the elongation decreased, particularly when the carbon exceeded 0.30 wt.%.
Corrosion testing of the MIM 316L stainless steel was done in different solutions (NaCl and HCl) (Fig. 1(b)). The corrosion resistance was found to decrease with increasing carbon content, particularly in the NaCl solution, with the corrosion rate increasing when carbon content exceeded 0.3 wt%. Nevertheless, the corrosion resistance of the MIM 316L stainless steel stents with a carbon content below 0.2 wt.% was found to be acceptable.
The authors stated that carbon combines with Cr to form carbide, which degrades the Cr content in the 316L stainless steel matrix and hence accelerates corrosion. In HCl solution, the corrosion rate is considerably higher than that in NaCl solution when the carbon content is not greater than 0.2 wt.%. However, beyond this, the corrosion rate in HCl solution is lower than that in NaCl solution. The authors plan further investigations to elucidate this mechanism. They also stated that because mechanical properties and corrosion resistance of 316L stainless steel are extremely sensitive to the fluctuation in the carbon content, this needs to be precisely controlled to satisfy the performance requirements of MIM vascular stents.
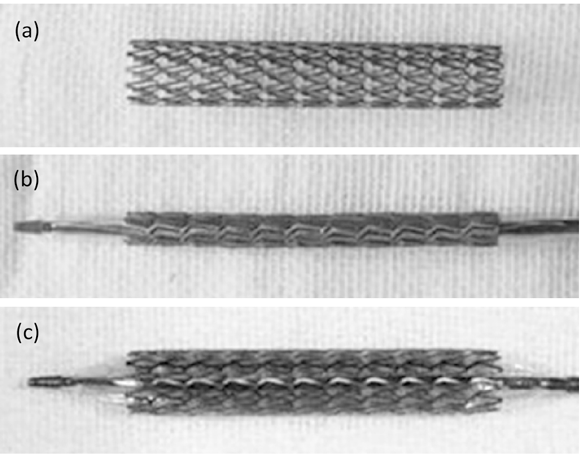
The authors used 316L stainless steel stents with ultra low carbon content having good corrosion resistance to produce the actual MIM stents shown in Fig. 2, which were used in in-vitro toxicity and in-vivo reliability studies. The as-received MIM stents exhibited good shape retention with no nodes, stains, cracks, or other defects on the surface. Some of the residual 316L stainless steel powder observed on the side surfaces of the stents (as can be seen in Fig. 3 (e) and (f)) were removed by an electrical polishing process.
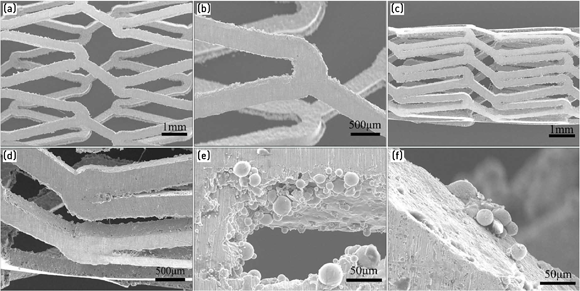
The MIM vascular stents showed a completely austenitic structure with grain sizes ranging from 20–100 μm. The composition and size of the grains on the outer layer were similar to those in the centre, which indicated that MIM technology could produce a completely homogenous microstructure.
In-vitro cytotoxicity testing to evaluate the safety of the MIM 316L stainless steel stents showed no obvious cell toxicity and the MIM stents were successfully implanted into the aortas of dogs. In-vivo biomedical behaviour of the MIM vascular stents using digital subtraction angiography (DSA) showed that no migration, twisting, collapse, infection, acute or chronic thrombosis, stenosis or obstruction of the stents was observed during the entire observation period of up to six months.
The results implied that the MIM stents showed good performance and biocompatibility; however, the authors stated that longer observation periods were still warranted. The authors concluded that compared with laser cutting and braiding, MIM technology considerably improves the utilisation of stent materials. To the best of their knowledge, this study is the first to validate the biocompatibility and safety of MIM vascular stents in experimental animals. However, they recommend that further studies be carried out in other material systems, as well as an investigation into mechanical fatigue reliability and biological safety.
www.journals.elsevier.com/transactions-of-nonferrous-metals-society-of-china

















